what prefix should be used to name a hydrocarbon that has an eight-carbon parent chain?
Nomenclature of Alkanes
- Page ID
- 857
The names of all alkanes cease with -ane. Whether or not the carbons are linked together end-to-end in a ring (chosen circadian alkanes or cycloalkanes) or whether they contain side chains and branches, the name of every carbon-hydrogen concatenation that lacks whatever double bonds or functional groups volition stop with the suffix -one.
Alkanes with unbranched carbon chains are simply named by the number of carbons in the chain. The get-go iv members of the serial (in terms of number of carbon atoms) are named every bit follows:
- CH4 = methane = ane hydrogen-saturated carbon
- C2H6 = ethane = two hydrogen-saturated carbons
- C3H8 = propane = three hydrogen-saturated carbons
- C4H10 = butane = four hydrogen-saturated carbons
Alkanes with five or more carbon atoms are named past calculation the suffix -ane to the appropriate numerical multiplier, except the concluding -a is removed from the basic numerical term. Hence, CfiveH12 is called pentane, Chalf-dozenH14 is called hexane, C7H16 is chosen heptane and then forth.
Straight-chain alkanes are sometimes indicated by the prefix n- (for normal) to distinguish them from branched-chain alkanes having the same number of carbon atoms. Although this is non strictly necessary, the usage is still mutual in cases where there is an important difference in properties between the straight-concatenation and branched-concatenation isomers: e.grand. north-hexane is a neurotoxin while its branched-chain isomers are non.
IUPAC classification
The IUPAC nomenclature is a system on which most organic chemists have agreed to provide guidelines to let them to acquire from each others' works. Nomenclature, in other words, provides a foundation of language for organic chemistry.
Number of Hydrogen to Carbons
This equation describes the relationship between the number of hydrogen and carbon atoms in alkanes:
- H = 2C + 2
where "C" and "H" are used to represent the number of carbon and hydrogen atoms present in one molecule. If C = ii, then H = 6.
Many textbooks put this in the following format:
- CnH2n+two
where "Cn" and "H2n+2" stand for the number of carbon and hydrogen atoms present in one molecule. If Cnorth = 3, then H2n+2 = ii(3) + two = viii. (For this formula look to the "n" for the number, the "C" and the "H" letters themselves exercise not change.)
Progressively longer hydrocarbon bondage can be made and are named systematically, depending on the number of carbons in the longest chain.
The following table contains the systematic names for the showtime twenty straight chain alkanes. It will be of import to familiarize yourself with these names because they will be the basis for naming many other organic molecules throughout your course of study.
Cartoon Hydrocarbons
Recall that when carbon makes iv bonds, it adopts the tetrahedral geometry. In the tetrahedral geometry, just two bonds can occupy a aeroplane simultaneously. The other two bonds point in back or in forepart of this plane. In guild to stand for the tetrahedral geometry in 2 dimensions, solid wedges are used to represent bonds pointing out of the plane of the drawing toward the viewer, and dashed wedges are used to correspond bonds pointing out of the airplane of the drawing away from the viewer. Consider the following representation of the molecule methyl hydride:
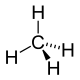
Figure 1: Two dimensional representation of methane
In the in a higher place drawing, the two hydrogens connected past solid lines, as well every bit the carbon in the middle of the molecule, exist in a plane (specifically, the airplane of the figurer monitor / piece of newspaper, etc.). The hydrogen connected by a solid wedge points out of this aeroplane toward the viewer, and the hydrogen connected past the dashed wedge points behind this airplane and away from the viewer.
In drawing hydrocarbons, it tin can exist time-consuming to write out each cantlet and bond individually. In organic chemical science, hydrocarbons can be represented in a shorthand annotation called a skeletal structure. In a skeletal construction, only the bonds between carbon atoms are represented. Individual carbon and hydrogen atoms are not fatigued, and bonds to hydrogen are not drawn. In the instance that the molecule contains just unmarried bonds (sp3 bonds), these bonds are fatigued in a "zig-zag" fashion. This is considering in the tetrahedral geometry all bonds betoken equally far abroad from each other as possible, and the construction is not linear. Consider the post-obit representations of the molecule propane:
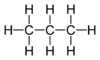
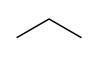
Figure ii: Full construction of propane and skeletal structure of propane
Only the bonds betwixt carbons have been drawn, and these have been drawn in a "zig-zag" manner. Notation that at that place is no representation of hydrogens in a skeletal structure. Since, in the absence of double or triple bonds, carbon makes iv bonds total, the presence of hydrogens is implicit. Whenever an insufficient number of bonds to a carbon cantlet are specified in the construction, it is assumed that the rest of the bonds are made to hydrogens. For instance, if the carbon atom makes only one explicit bond, in that location are three hydrogens implicitly attached to it. If information technology makes two explicit bonds, there are ii hydrogens implicitly attached, etc. Note also that two lines are sufficient to represent three carbon atoms. Information technology is the bonds only that are being drawn out, and it is understood that in that location are carbon atoms (with three hydrogens fastened!) at the terminal ends of the structure.
Alkyl Groups
Alkanes can be described by the general formula CnH2n +2. An alkyl group is formed by removing one hydrogen from the alkane chain and is described by the formula CnH2n +i. The removal of this hydrogen results in a stalk change from -ane to -yl. Have a look at the following examples.
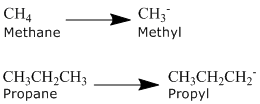
The same concept can exist applied to any of the straight chain alkane names provided in the table above.
| Name | Molecular Formula | Condensed Structural Formula |
|---|---|---|
| Methyl hydride | CH4 | CHfour |
| Ethane | C2H6 | CH3CH3 |
| Propane | C3H8 | CHiiiCH2CH3 |
| Butane | C4H10 | CH3(CH2)2CH3 |
| Pentane | C5H12 | CHiii(CH2)iiiCH3 |
| Hexane | C6H14 | CH3(CH2)4CH3 |
| Heptane | C7H16 | CH3(CH2)5CHthree |
| Octane | C8H18 | CHthree(CH2)sixCHthree |
| Nonane | C9Htwenty | CH3(CH2)7CHthree |
| Decane | C10H22 | CHiii(CH2)8CH3 |
| Undecane | C11H24 | CHthree(CH2)9CHiii |
| Dodecane | C12H26 | CH3(CH2)xCH3 |
| Tridecane | CxiiiH28 | CH3(CH2)11CH3 |
| Tetradecane | C14Hxxx | CHthree(CH2)12CH3 |
| Pentadecane | C15H32 | CH3(CH2)13CH3 |
| Hexadecane | CxviH34 | CH3(CH2)14CH3 |
| Heptadecane | C17H36 | CHiii(CH2)xvCH3 |
| Octadecane | C18H38 | CH3(CHii)xviCH3 |
| Nonadecane | C19H40 | CHthree(CH2)17CH3 |
| Eicosane | C20H42 | CH3(CH2)18CH3 |
Using Common Names with Branched Alkanes
Certain branched alkanes have common names that are yet widely used today. These mutual names make use of prefixes, such equally iso- , sec- , tert- , and neo- . The prefix iso- , which stands for isomer, is unremarkably given to ii-methyl alkanes. In other words, if in that location is methyl grouping located on the 2d carbon of a carbon concatenation, we tin employ the prefix iso- . The prefix will be placed in forepart of the alkane proper noun that indicates the total number of carbons. Examples:
- isopentane which is the same as 2-methylbutane
- isobutane which is the aforementioned equally ii-methylpropane
To assign the prefixes sec- , which stands for secondary, and tert- , for tertiary, it is important that we first larn how to classify carbon molecules. If a carbon is attached to simply one other carbon, it is called a primary carbon. If a carbon is fastened to two other carbons, it is called a seconday carbon. A tertiary carbon is attached to three other carbons and last, a quaternary carbon is attached to iv other carbons. Examples:
- iv-sec-butylheptane (30g)
- 4-tert-butyl-five-isopropylhexane (30d); if using this instance, may want to move sec/tert after iso disc
The prefix neo- refers to a substituent whose 2d-to-last carbon of the chain is trisubstituted (has three methyl groups attached to it). A neo-pentyl has 5 carbons total. Examples:
- neopentane
- neoheptane
Alkoxy Groups
Alkoxides consist of an organic group bonded to a negatively charged oxygen atom. In the general form, alkoxides are written as RO-, where R represents the organic substituent. Similar to the alkyl groups above, the concept of naming alkoxides tin exist applied to any of the straight chain alkanes provided in the table above.
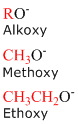
Three Principles of Naming
- Cull the longest, near substituted carbon chain containing a functional grouping.
- A carbon bonded to a functional group must take the lowest possible carbon number. If there are no functional groups, and then any substitute present must have the everyman possible number.
- Take the alphabetical club into consideration; that is, after applying the offset 2 rules given above, brand sure that your substitutes and/or functional groups are written in alphabetical order.
Example ane
What is the name of the following molecule?
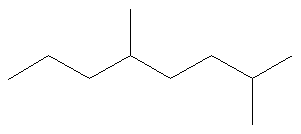
SOLUTION
Dominion #one: Choose the longest, about substituted carbon chain containing a functional group. This example does not incorporate any functional groups, so we only need to exist concerned with choosing the longest, most substituted carbon concatenation. The longest carbon chain has been highlighted in red and consists of eight carbons.
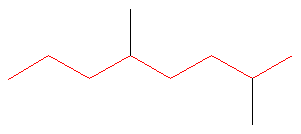
Dominion #2: Carbons bonded to a functional group must have the lowest possible carbon number. If at that place are no functional groups, then any substitute present must take the lowest possible number. Because this example does not contain any functional groups, we only need to be concerned with the two substitutes nowadays, that is, the ii methyl groups. If nosotros begin numbering the concatenation from the left, the methyls would exist assigned the numbers 4 and 7, respectively. If nosotros begin numbering the chain from the right, the methyls would be assigned the numbers 2 and five. Therefore, to satisfy the second rule, numbering begins on the right side of the carbon chain as shown below. This gives the methyl groups the lowest possible numbering.
Rule 3: In this example, in that location is no need to use the third rule. Because the ii substitutes are identical, neither takes alphabetical precedence with respect to numbering the carbons. This concept will become clearer in the following examples.
Example ii
What is the name of the following molecule?
SOLUTION
Rule #one: Choose the longest, well-nigh substituted carbon concatenation containing a functional group. This example contains two functional groups, bromine and chlorine. The longest carbon chain has been highlighted in red and consists of seven carbons.
Rule #ii: Carbons bonded to a functional grouping must have the lowest possible carbon number. If there are no functional groups, then whatsoever substitute present must have the lowest possible number. In this example, numbering the chain from the left or the right would satisfy this rule. If we number the chain from the left, bromine and chlorine would be assigned the second and sixth carbon positions, respectively. If we number the chain from the right, chlorine would exist assigned the second position and bromine would be assigned the 6th position. In other words, whether we cull to number from the left or right, the functional groups occupy the second and sixth positions in the chain. To select the correct numbering scheme, we need to use the third dominion.
Dominion #3: After applying the first 2 rules, accept the alphabetical order into consideration. Alphabetically, bromine comes before chlorine. Therefore, bromine is assigned the second carbon position, and chlorine is assigned the sixth carbon position.
Case iii
What is the name of the follow molecule?
SOLUTION
Dominion #1: Choose the longest, about substituted carbon concatenation containing a functional group. This example contains two functional groups, bromine and chlorine, and one substitute, the methyl grouping. The longest carbon chain has been highlighted in scarlet and consists of vii carbons.
Dominion #2: Carbons bonded to a functional group must have the everyman possible carbon number. After taking functional groups into consideration, any substitutes present must have the lowest possible carbon number. This particular example illustrates the point of difference principle. If we number the chain from the left, bromine, the methyl group and chlorine would occupy the 2nd, fifth and sixth positions, respectively. This concept is illustrated in the 2d drawing below. If we number the chain from the right, chlorine, the methyl group and bromine would occupy the 2d, third and sixth positions, respectively, which is illustrated in the first drawing below. The position of the methyl, therefore, becomes a indicate of difference. In the first drawing, the methyl occupies the third position. In the second drawing, the methyl occupies the fifth position. To satisfy the second dominion, we want to choose the numbering scheme that provides the everyman possible numbering of this substitute. Therefore, the first of the two carbon chains shown beneath is right.
Therefore, the showtime numbering scheme is the appropriate one to use.
Once you have determined the right numbering of the carbons, it is often useful to make a listing, including the functional groups, substitutes, and the name of the parent chain.
Dominion #3: After applying the kickoff two rules, take the alphabetical guild into consideration. Alphabetically, bromine comes before chlorine. Therefore, bromine is assigned the second carbon position, and chlorine is assigned the 6th carbon position.
Parent chain: heptane 2-Chloro 3-Methyl 6-Bromo
6-bromo-2-chloro-3-methylheptane
Problems
What is the name of the follow molecules?
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_%28Organic_Chemistry%29/Alkanes/Nomenclature_of_Alkanes
0 Response to "what prefix should be used to name a hydrocarbon that has an eight-carbon parent chain?"
Enviar um comentário